Despite rapidly rising rates of breast cancer in younger women and overwhelming evidence supporting the efficacy and cost-effectiveness of screening, the Canadian Task Force on Preventive Health Care has recommended against systematic screening for women between the ages of 40 and 49.
The decision to not endorse breast cancer screening for young women is perplexing and dangerous, given that early detection is critical in the fight against breast cancer.
The Canadian Cancer Society and the United States Preventive Services Task Force have already changed their recommendations for breast screening, while most provincial governments in Canada have implemented or committed to breast screening starting at age 40.
Yet, it appears that the Task Force’s decision may have been a foregone conclusion, with leadership expressing reluctance to alter guidelines even before the process began.
These views were reinforced in a recent publication in Canadian Family Physician in which members of the Task Force dismissed the efficacy of screening and evolving treatments.
Focusing on potential harms and old data
The Task Force disproportionately focuses on the potential harms of screening, such as over-diagnosis (the diagnosis of a cancer that would never have caused problems for an individual in their lifetime) and anxiety around imaging callbacks, while minimizing the undeniable benefits of saving lives and reducing suffering through early detection.
While randomized controlled trials (RCTs) are the gold standard for guideline formulation, the reliance of the Task Force on 30- to 60-year-old outdated RCTs fails to account for modern diagnostic and treatment advancements. The guideline process was paused in the fall, with experts demanding that only evidence from after 2000 be considered. However, this advice was ignored, and the Task Force continued to disproportionately prioritize aged RCTs and undervalue recent observational trials, which better align with present screening and treatment norms.
This diminishes the perceived benefit of screening, as the mortality benefit of screening in RCTs is 15 per cent, compared to 53 per cent in observational trials. The Task Force also tells only part of the story of the benefits of screening by framing its advantages within a limited time span (10 years from entry into screening), and not manifesting the accrued benefits over an individual’s entire lifetime.
Moreover, the Task Force’s approach to risk assessment is alarmingly simplistic, disregarding individual factors, such as race and ethnicity, breast density and family history. These significantly influence breast cancer risk.
But by applying a one-size-fits-all model, they risk misinforming women about their individual risk and denying them access to potentially life-saving screenings, especially racialized women who are significantly more likely to be diagnosed in their 40s.
Perhaps most concerning is the Task Force’s contention that many cancers identified through screening would not progress or would regress naturally, leading to unnecessary treatments or “over-diagnosis.” This flawed reasoning ignores the reality of untreated cancers that most certainly progress, and disregards medical innovations such as genomic recurrence risk profiling that allow for tailored de-escalation of treatment, minimizing over-treatment.
Withholding screening based on an unproven theory that a cancer may never cause a problem, when screening provides a clear mortality benefit, is unjustifiable.
Access to care
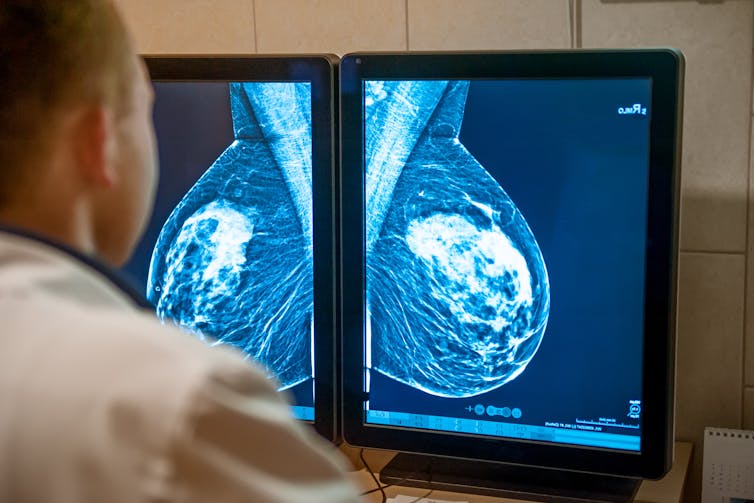
(Shutterstock)
The Task Force couches all of its recommendations in “shared decision-making,” urging patients and primary-care providers to collaborate to establish what is right for each individual patient.
In theory this concept is admirable. However, family physicians look to the Task Force for guidance on how to counsel their patients, and when they receive inaccurate information about the harms and benefits of screening, the power imbalance between provider and patients undermines the informed and shared nature of decision-making. Consequently, women’s access to vital health-care options are limited, perpetuating disparities in breast cancer outcomes.
If the harms from treatments faced by individuals are not compelling enough, there is also a financial cost to delaying a breast cancer diagnosis. Work done by researchers in Ottawa showed that the costs of breast cancer management in Ontario vary significantly by stage at diagnosis. From $14,505 for ductal carcinoma in situ (DCIS, a very early stage of breast cancer), to median $39,263, $76,446, $97,668 and $370,398 for Stage I, II, III and IV respectively. In a single-payer public health-care system, these costs significantly outweigh the cost of screening mammography.
The updated Task Force guidelines have cast the available science to minimize the benefits of screening and amplify the harms, ultimately denying women access to screening. We believe the consequences of these new guidelines could be dire, with many young women paying with their lives.
The majority of provinces and territories have acknowledged these shortcomings, ignoring the existing guidelines, and allowing self-referral for screening for women 40-49, understanding that “shared decision-making” creates barriers for women to access screening, especially in this era of uneven access to primary care.
How can it be that the Canadian Cancer Society, the United States and nine provinces in Canada support screening in women 40-49, but our national guidelines do not? Clearly these other bodies have found the data compelling enough to offer screening, so how is it that the Task Force can be the lone voice finding that the harms outweigh the benefits?
We believe the new breast cancer recommendations are misguided, and should be viewed as a cautionary tale for all Canadian preventive health. If we do not pivot to heed the call of reason and begin to use only the latest evidence reflecting the advances in treatment and diagnostics used every day in our clinics, we risk lagging behind other countries and witnessing a decline in our cancer outcomes.
As cancer clinicians and researchers, none of us would want to be a 40-year-old woman in Canada with these guidelines, given the late-stage incurable breast cancers we see on the oncology wards that could have potentially been prevented by screening.
![]()
Anna N. Wilkinson is a consultant for Thrive Health.
Jean Seely receives funding from Canadian Clinical Trials Group to lead the TMIST trial in Ottawa. The funds cover the research staff to perform the study.
Moira Rushton and Suleena Duhaime do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.