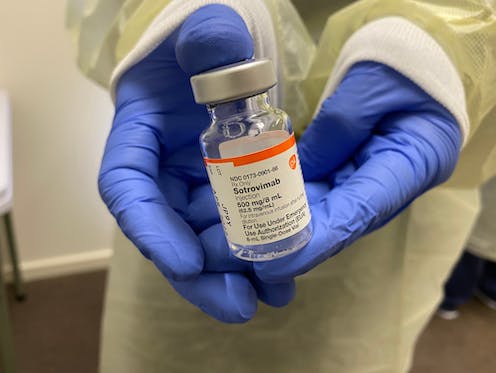
New guidance from the World Health Organization (WHO) strongly advises against using the antibody therapies sotrovimab and casirivimab-imdevimab to treat patients with COVID-19.
This guidance, published in the British Medical Journal, replaces previous conditional recommendations for the use of these drugs. It’s based on emerging evidence that they’re not likely to work against current COVID variants such as omicron.
This means that, at least for the time being, there are no recommended antibody therapies to treat COVID. There are, however, still other treatment options. Let’s take a look.
We know that severe COVID is driven by collateral damage from our own immune system. Some of the most effective COVID treatments are anti-inflammatory medicines, which reduce exaggerated immune responses against the virus. Strong evidence continues to support the use of drugs such as corticosteroids, anti-IL-6 and baricitinib.
Separate to anti-inflammatory drugs, we have two types of therapies that directly target SARS-CoV-2, the virus that causes COVID-19. These are antiviral drugs and antibody treatments.
Antiviral drugs allow the virus to enter our cells but prevent it from replicating, thereby reducing the impact of an infection.
Remdesivir, which was originally developed for hepatitis C, retains efficacy against omicron sub-variants BA.2.12.1, BA.4 and BA.5 in the lab. In the new guidance the WHO has conditionally recommended remdesivir to treat patients with severe COVID, but has advised against its use for patients who are critically unwell, based on results from a series of recent randomised trials.
Other antivirals include molnupiravir, which the WHO continues to conditionally recommend, and nirmatrelvir and ritonavir (a combination known as Paxlovid), which is recommended strongly. These drugs are taken orally, while remdesivir is administered intravenously.
Read more:
COVID: antiviral drugs are a vital weapon – but misusing them could backfire
Meanwhile, antibody therapies work by coating a protein on the surface of SARS-CoV-2, called the spike protein, thereby blocking the virus from entering human cells. They can also help eliminate infected cells which have been hijacked by the virus.
Sotrovimab is one such antibody therapy. It’s a monoclonal antibody, which means it only targets a specific region of the virus’s spike protein. In clinical trials conducted before the omicron variant emerged, sotrovimab reduced the risk of disease progression.
This led to its emergency authorisation by the US Food and Drug Administration and the UK’s Medicines and Healthcare products Regulatory Agency in 2021.
Table of Contents
So what’s changed?
A key challenge that comes with using monoclonal antibodies to manage SARS-CoV-2 infections is that they only bind to a single region of the spike protein. As the virus evolves, this region of the protein that the antibodies recognise can be altered by mutations. So it’s not entirely surprising that lab studies suggest the emergence of omicron has diminished sotrovimab’s efficacy.
Casirivimab-imdevimab combines two monoclonal antibodies, thereby targeting two different regions of the spike protein, to try to overcome the speed at which SARS-CoV-2 can change. But this combination has proven ineffective in preventing omicron infection in lab experiments, leading the WHO to change its advice.

Design_Cells/Shutterstock
Evidence will evolve alongside the virus
Regulatory agencies and the WHO keep a close eye on the way existing treatments respond to emerging variants, and issue prescribing recommendations accordingly.
For drugs such as remdesivir that have a modest impact in certain groups of patients, the WHO issues conditional recommendations. Drugs that continue to work consistently receive strong recommendations, but these are also subject to review as the virus evolves.
While it might seem alarming that the WHO has changed its mind on these two antibody treatments, it’s actually a sign that the scientific process is working as it should.
This is now the 12th iteration of the WHO living guideline, and the advice on the provision of COVID treatments is likely to continue to be updated as the pandemic plays out.
Who will be most affected?
In the fight against infection, we are not all equal. Vaccination has significantly reduced the risk of severe COVID for the vast majority of the population. However, some people are born with deficient immune systems or receive treatments that weaken their immune responses later in life, for example after receiving an organ transplant or chemotherapy. Certain infections or chronic diseases can further damage the immune system, which also naturally weakens with age.
One of the most common forms of immune deficiency is an inability to produce enough antibodies following vaccination or infection. So antibody therapies, which seek to supplement or replace those antibodies artificially, stand to benefit many people who are immunocompromised in particular.
Read more:
COVID: WHO recommends two new treatments – here’s how they work
While guaranteeing monoclonal antibodies remain effective against a rapidly changing virus is an enormous challenge, this isn’t necessarily the end of this type of treatment for COVID. Next-generation monoclonal antibodies that better neutralise omicron subvariants may well be identified, although these too are unlikely to remain effective for long.
For the immunocompromised, but also for the wider public, there is an ongoing need for continued research into, and access to, effective COVID treatments – antivirals, antibodies and otherwise.
Unfortunately, when dealing with RNA viruses, mutations can rapidly bring down our defences. To prolong efficacy, combination treatments will be an important way forward compared with single-agent therapies.
![]()
Dr Zania Stamataki receives funding from the Medical Research Foundation, Innovate UK and BCHRF and she shares a PhD student with AstraZeneca on an iCASE MRC UKRI studentship unrelated to the article topic.
Adrian Shields receives funding from Association of Clinical Biochemistry and Laboratory Medicine and is a co-investigator on the UKRI/MRC funded COV-AD study.























