One in four adults in the UK are obese. Obesity is associated with many health conditions including high blood pressure, type 2 diabetes, heart attacks, stroke and cancer. The financial cost of obesity to the NHS is an estimated £6 billion a year.
In an effort to reduce the burden of obesity on the NHS and cut waiting times, the UK government has announced a £40 million pilot scheme that will explore ways of making the obesity drug Wegovy available to many more patients living in the UK.
In February 2023, the National Institute for Health and Care Excellence (Nice) recommended the use of Wegovy for adults with a BMI above 35 and at least one weight-related disease, such as diabetes or heart disease. Eligible patients would need a prescription to access these drugs, and prescriptions could only be handed out by practitioners after patients had attended a specialist weight management clinic.
Under the new pilot scheme, however, it’s hoped these drugs can also be prescribed by GPs and specialist pharmacies. This would remove the long wait for a clinical appointment, making the drug available to thousands more patients in England.
Table of Contents
How does Wegovy work?
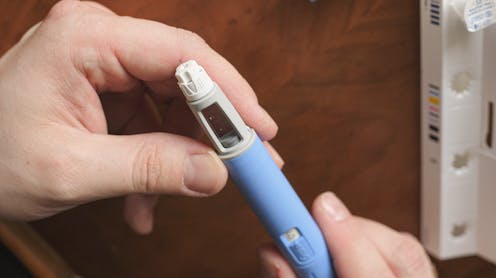
Wegovy is the brand name of semaglutide – a drug that mimics a naturally-occurring hormone in the body which is released in the intestines after we eat.
The hormone signals the brain to stop feeling hungry. Semaglutide mimics it, reducing a person’s hunger levels and making it easier for them to lose weight. Semaglutide also improves blood sugar levels, which is why they were originally introduced to treat diabetes.
According to the results of a major clinical trial, weight loss can begin immediately after taking semaglutide. However, the study also found that weight loss plateaued about a year after starting, after participants had lost around 15% of their body weight – despite continued use.
Importantly, all participants were also given advice on maintaining a healthy diet, and advised to exercise for 150 minutes a week.
Are there any downsides?
Semaglutide has some side-effects, with nausea being one of the most common. This is less likely to happen at lower doses of the drug. Other potential side effects include diarrhoea or constipation, headache and tiredness.
Another pitfall of using semaglutide to manage obesity is whether and when to stop it. At the moment, Nice has approved the drug for a maximum of two years’ use. While it does not report on its decision-making process, this time restriction is likely due to concerns about the long-term cost of the drug, as well as a lack of data for long-term use.
Another problem with taking semaglutide to manage obesity is that some weight regain, after stopping the drug, is nearly inevitable. In fact, research shows people regain most of the weight they lost on semaglutide within six to 12 months of stopping.

J.Thasit/ Shutterstock
And while current evidence shows the drug is safe, we don’t yet know how safe it is for long-term use – especially since the doses to treat obesity are roughly twice as strong as what is needed for diabetes. Ongoing safety monitoring is crucial.
It’s also worth noting that GLP-1RA drugs such as semaglutide are not cheap. The cost to the NHS (for lower doses to treat diabetes) is approximately £75 for a pack of four pens. People who purchase the drugs (after a healthcare recommendation outside of the NHS) will likely pay much more.
The cost of the drugs to the NHS must be balanced against the potential economic benefits of weight loss. But calculating this is complicated, since obesity affects so many areas of physical and mental health.
Will the pilot scheme be helpful?
To date, obesity management in the UK has been a tiered service.
Tier 1 includes public health lifestyle messages – for example, health promotion from primary care such as your GP or local pharmacy. Tier 2 consists of group-based diet support. Tiers 3 and 4 are mostly hospital-based and include the provision of weight-loss drugs or bariatric surgery. At the moment, access to tiers 3 and 4 can be slow.
The NHS also suffers from a fragmentation of services, with funding and care not spread equally throughout the country – meaning people living in some regions may be less likely to access treatment. The new pilot scheme would streamline access to weight-loss medications, making care more equal in all parts of the country.
While this could have many benefits for patients, these proposals will only work if the right measures are put in place alongside them.
Guidance and continued support for patients on making and maintaining health lifestyle changes during and after taking Wegovy will be essential. Data shows that weight loss with GLP-1RA drugs is greater if taken as part of a supported, holistic change to diet and exercise. Support to maintain these lifestyle changes after stopping semaglutide will also be important as it may help reduce weight regain.
Accessibility to weight-loss drugs such as Wegovy is an important component of an overall obesity strategy – but it cannot, in and of itself, resolve the obesity crisis. While the UK government is already taking some action to tackle the drivers of obesity (such as introducing calorie labelling on menus and restricting the location of unhealthy foods in shops), these policies may not do enough.
Without effective implementation of a range of lifestyle-related policies alongside roll-out of the pilot scheme, it’s likely that expanding access to Wegovy may not have as great an effect as hoped.
![]()
Martin Whyte has received investigator-led research funding from AstraZeneca, Eli Lilly and Sanofi and speaker fees from Amarin UK